
Water Vs Battery H2 And O2 Out From Water Water E Water vs battery || h2 and o2 out from water || water electrolysis gas #science #experiment#expriment #experiment #science #scienceexperiment #waterelevator. Electrolysis of water by providing energy from a battery, water (h 2 o) can be dissociated into the diatomic molecules of hydrogen (h 2) and oxygen (o 2). this process is a good example of the the application of the four thermodynamic potentials. the electrolysis of one mole of water produces a mole of hydrogen gas and a half mole of oxygen gas.
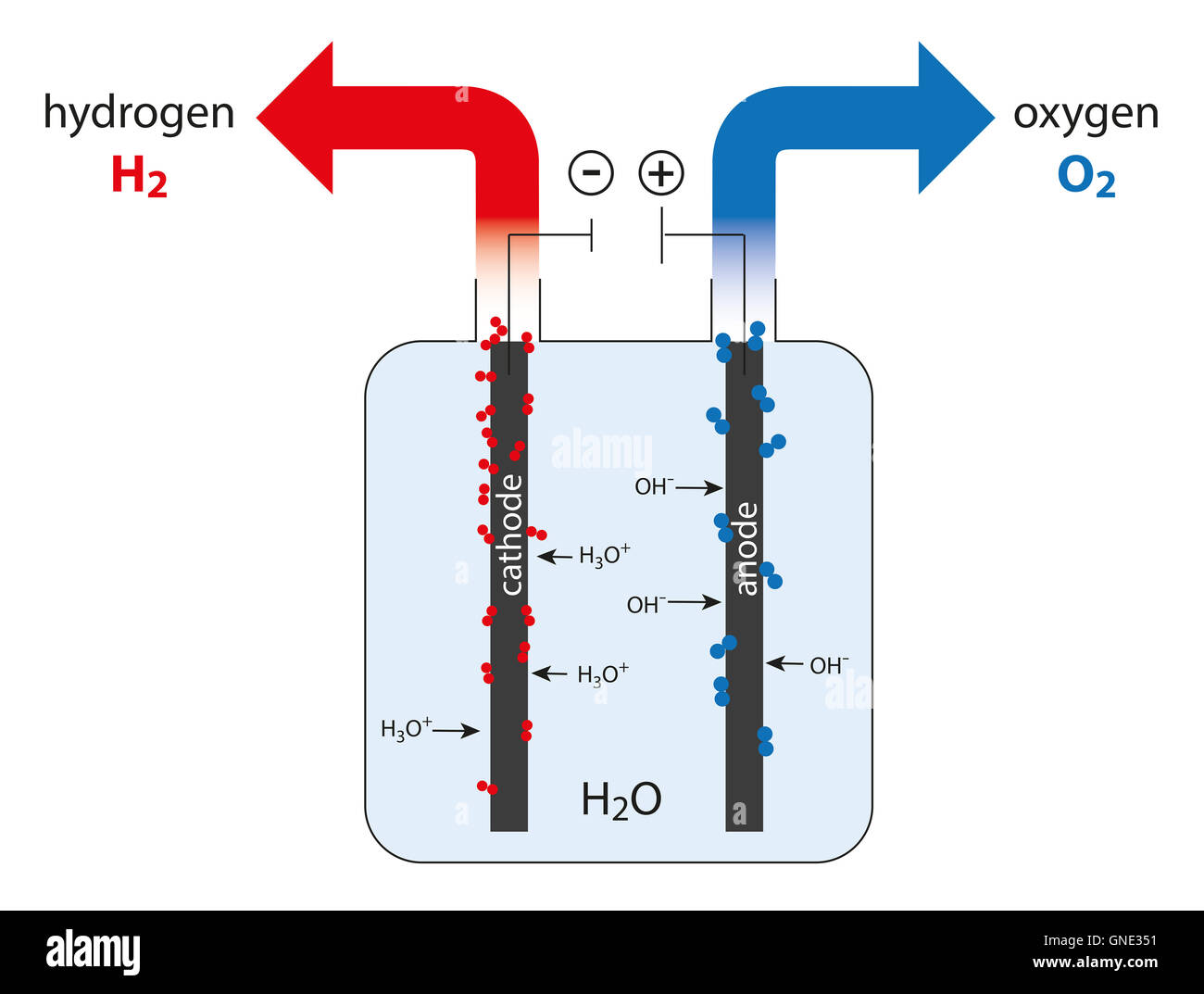
Water Electrolysis Cell Thermodynamics. [] the electrolysis of water in standard conditions requires a theoretical minimum of 237 kj of electrical energy input to dissociate each mole of water, which is the standard gibbs free energy of formation of water. it also requires thermal energy to balance the change in entropy of the reaction. Hydrogen gas—involving the formation of water—is exothermic. the energy released by formation of h–o bonds in water molecules is greater than that required to break bonds in h 2 and o 2 molecules: 2 h 2 o 2 2 h 2 o energy therefore, the separation of water into its elements—the reverse reaction—must be endo. Electrolysis of water. the electrolysis of water produces hydrogen and oxygen gases. the electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. the electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Electrolysis of water. the first thing we will need to do electrolysis of water is a battery with an estimated potential of greater than 1.23 v as the estimated cell potential is 1.23 v (see later). we are also going to need two electrodes (anode and cathode) which, unlike galvanic cells, are typically made of the same metal as it is not part.

Electrolysis Definition Reaction Process Lesson Study Electrolysis of water. the electrolysis of water produces hydrogen and oxygen gases. the electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h2so4 h 2 so 4 has been added. the electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Electrolysis of water. the first thing we will need to do electrolysis of water is a battery with an estimated potential of greater than 1.23 v as the estimated cell potential is 1.23 v (see later). we are also going to need two electrodes (anode and cathode) which, unlike galvanic cells, are typically made of the same metal as it is not part. Electrolysis of water experiment. energy is stored in the bonds of molecules. when these bonds split apart, the energy released can be used to do work. breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy, which can be turned into useful electricity to power our homes and cars. Electrolysis: splitting water teacher version in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to produce gases (like hydrogen and oxygen in the case of water). you will measure the volumes of gas produced and compare this to the predicted ratios from chemical.
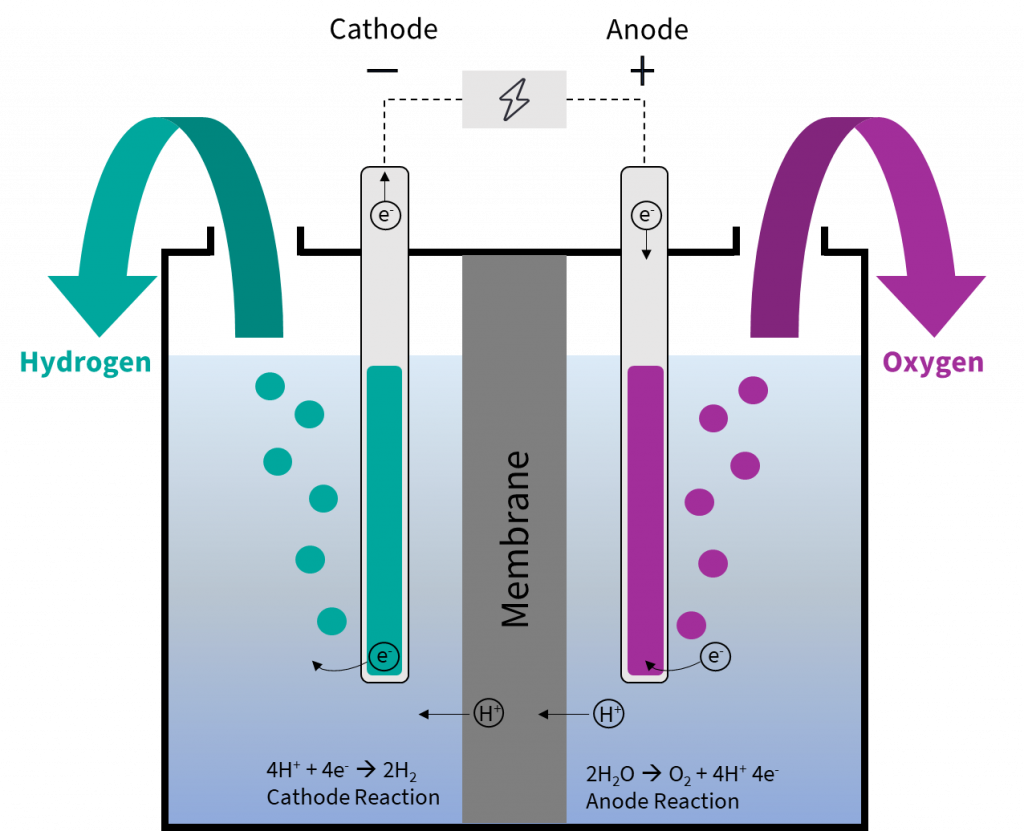
Water Electrolysis Explained вђ The Basis For Most Power To X Processes Electrolysis of water experiment. energy is stored in the bonds of molecules. when these bonds split apart, the energy released can be used to do work. breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy, which can be turned into useful electricity to power our homes and cars. Electrolysis: splitting water teacher version in this lab you will use a battery to perform electrolysis, or chemical decomposition, of different aqueous solutions (like water) to produce gases (like hydrogen and oxygen in the case of water). you will measure the volumes of gas produced and compare this to the predicted ratios from chemical.

Separate Hydrogen And Oxygen From Water Through Electrolysis 7 Steps

Comments are closed.